Rocket lab world records lead to a rocketing understanding of the mole
- Brennan Koch
- Nov 21, 2025
- 4 min read
Kids love rockets.
Kids hate math.
Kids love competing.
Kids hate calculating.
The rocket lab keeps the things that kids love, and then surprises kids with the satisfaction of the things they hate. It is my favorite lab of the year, making hydrogen and oxygen rockets and launching them down the hall. But the real magic happens the day after the lab. I wrote a blog about the lab setup a couple years ago. You can check it out here. I’m going to focus on what can be learned from the data (you know, the stuff that the kids hate).
Competition leads to data
I love to hype up the competitive aspect of this lab. I announce for every period there will be a class record. And then… drumroll… there is the world record. I have kept records for about eight years now. So when I lay out the tape measure in the hall, I put an Erlenmeyer flask with a little flag in it all the way down at the world record. That is the ultimate goal! This year it actually happened! Two students broke the world record. Now their names will be etched in history forever. You know what else got etched? Data. And lots of it.

The students record their percent hydrogen and the distance the rocket travels. Over the hundreds of shots, we get a lot of class data, which I pool to use the day after the lab. They send me their data and I put it into a class excel spreadsheet. I used to have each group do their own spreadsheet, but to really get decent data, it takes a whole lot of points! Plus, I can spend more time helping them interpret the data rather than slogging through exponential trendlines.
Create a trendline
By this point in the year, my students are familiar with trendlines. I put the data on board and have them describe what the trendline might look like. They have only used linear trends, so they don’t think it’s a very good trend. Then I show them the parabolic trend line. It fits much better! I put the equation on the graph. And now for the first big moment. I ask them find the perfect percent hydrogen. They can do it visually. They point to the top of the parabola. No. I want the mathematical answer. How do I calculate the X value at the maximum of the parabola. It takes a minute, but someone will sheepishly answer, -b/2a. Wait! What? Math actually did something? All of a sudden, the old quadratic equation seems a little cooler. And they really want to go shoot a rocket again with their new-found knowledge.
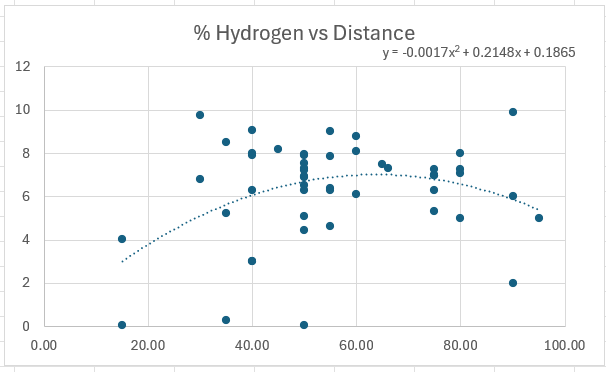
Most classes get the maximum on their graph close to the right answer (66%). But there will be times that it doesn’t. When a class gets bad data, I show them their data. And then add it to the all-school data and we interpret that instead.
What does that mean about moles?
I have introduced moles through playing Up & Atom. You can see a blog about that here. They have some good estimation skills, but they don’t have a deep understanding of what the mole means in the real world… yet. We discuss what the 66% means. That means there are two parts of hydrogen for every one part of oxygen. That could mean that for every oxygen atom that reacted, two hydrogen atoms reacted. Or… those could be moles! Every mole of oxygen that reacts needs two moles of hydrogen!
We look at the reaction on the board. There is much slapping of foreheads, as they feel they should have known all along. There was an obvious mole ratio right in front of them! That second big moment, locks in their heads that there is a real, tangible effect of moles in compounds. Moles make rockets go farther! That must mean that moles do a lot of things. Or maybe even, everything.
Link moles to liters
So far, my students have only linked moles to atoms and mass. Now we get to start the conversation about moles of gas at STP. 1 mole of ANY gas has 22.4L of volume at standard conditions. We can connect the idea that if we pump twice the volume of hydrogen into the rocket that there are twice the number of moles of gas. It makes for a pretty seamless transition.
At the end of the second day of the lab, the kids start to realize that there is a direct link between the real world of rockets and world records and the mysterious world of the mole in chemistry. Real links help real kids really understand.
Want to introduce moles with an engaging group activity followed by a strategic game where kids convert among moles, grams, and atoms? Try Up & Atom! It's our #1 selling game.





Comments